- europages
- >
- Import-export - medical and surgical equipment
- >
- MAXCERT LTD
- >
- MDR Technical Documentation
MDR Technical Documentation
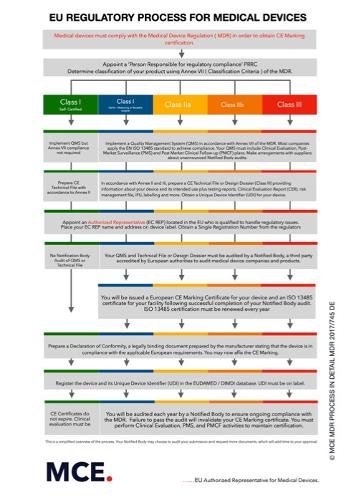
Description
Our experienced team has for years done documentation for companies who are manufacturers of medical devices /surgical instruments. We provide Quality-Management-Systems for meeting legal and normative requirements. For manufacturers of medical devices / surgical instruments class I (including Is, Im, and new for MDR class Ir), IIa, and IIb we offer the following services: Implementation DIN EN ISO 13485 and the EU-Regulation 2017/745 MDR and 21 CFR 820 QSR Creating QM-Systems with procedural instructions and work instructions Technical Documentation Clinical evaluation Risk Management / Risk Analysis Essential Safety and Performance Requirements Validation of processes UDI-labeling Consulting for Regulatory Affairs Internal audits and external supplier audits Accompaniment and support with inspections by authorities and Audits by notified bodies Communication with authorities
- Import-export - medical and surgical equipment
- Technical documentation
- EUMDR
- iso 13485
Similar products
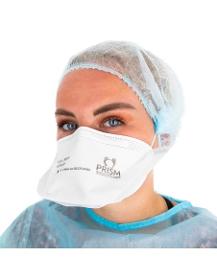
PRISM
France
• FFP2 half mask, non-reusable (NR), duckbill type. • Total back length: 225 mm= 8,85827 in, small size. • This FFP2 mask for personal respiratory protection is single use & non-sterile. • PRISM masks are manufactured on our production line in Frontignan in the Hérault region of France & meet the recommended European and French standards. • Double certification : - NF EN 149+A1 2009 ; - 14683 + AC 2019. • PRISM carries out quality controls as it produces. • COMPOSITION 1/ A "duckbill" mask organised in several layers: - the outer layers in non-woven polypropylene (= spunbond) ; - the two intermediate layers ensure filtration with meltblown fabric. 2/ A double nose bar to adjust the mask to the shape of the nose & to reduce fogging of the glasses. 3/ A double flat elastic band (manual separation) to secure the mask to the face.
Request for a quote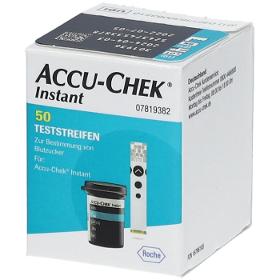
COMFORTPAT B.V.
Netherlands
Accu Chek Instant test strips For determining blood sugar Suitable for self-application Suitable for the following blood glucose meters: Accu-Check® Instant Accu-Check® Instant S
Request for a quote
AAAGILER GMBH
Germany
The calibrated, precise and medical scales from KERN & SOHN offer the highest precision and reliability for various applications in the medical and veterinary sector. Discover our comprehensive product range, which has been specially developed to meet the highest requirements in the healthcare sector, e.g. in hospitals, doctors' surgeries, nursing homes and pharmacies as well as for midwives.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
Stent manufacturing applies to a wide variety of medical applications including cardiovascular applications, birth control, kidney stone pain control and esophageal and gastrointestinal uses.
Request for a quoteRequest for quotes
Create one request and get multiple quotes form verified suppliers.
- Only relevant suppliers
- Data privacy compliant
- 100% free